Physics Today, volume 56, Number 7, page 24, July 2003
Experiments
Vindicate a 50-Year-Old Explanation of How Liquid Metals Resist Solidification
Diffracted x rays reveal a sequence of structural changes in a levitated drop of metal as it cools and freezes.
A metal's density barely falls on melting.
One might guess, therefore, that metal atoms in the liquid phase pack together
with almost the same efficiency and with almost the same order as in the solid
phase. And--to continue this line of speculation--if one tried to cool a liquid
metal below its equilibrium melting point, the few disorderly atoms would
easily fall into line with the ordered majority and the liquid would promptly
solidify.
In fact, as David Turnbull and Robert Cech showed in 1950, liquid metals can be cooled tens to
hundreds of degrees below their equilibrium melting temperatures without
solidifying.1 The trick is to prevent any
impurities or other extraneous components from nucleating the nascent solid.
According to classical nucleation theory,
a liquid solidifies when thermal fluctuations push it over an energy barrier.
This nucleation barrier, W, depends on ΔG, the difference
between the free energy of the liquid and solid phases. Specifically,
W ~ ΔG-2. As a liquid cools, ΔG
increases and lowers the nucleation barrier.
But classical theory also has W
proportional to γ3, where γ is the energy of the interface
between the liquid and solid phases. Turnbull and Cech
could undercool their samples because, for metals,
the interfacial energy is far higher than one might expect based on density
alone.
In 1952, to account for the unexpectedly
large γ, Charles Frank put forward a now classic hypothesis.2 It's possible to undercool
metals, he argued, because of a fundamental mismatch in the way atoms arrange
themselves in the liquid and solid phases.
According to Frank, atoms in the liquid
possess a short-range order based on the icosahedron.
One of Plato's perfect solids, the icosahedron has 20
triangular faces.
Frank picked icosahedral
order because it's among the tightest and least energetic ways to arrange a
small number of atoms. But because of their fivefold symmetry, icosahedral clusters can't combine to form a regular
crystal. Frank saw that the energy cost of creating an interface between such
structurally incompatible phases would be high.
When Frank published his paper, he didn't
know about quasicrystals, some of which possess icosahedral order. But if he had known about them, he might
have proposed the following test of his hypothesis:
Identify a material that has both a metastable quasicrystalline phase
and a stable crystalline phase. Melt the material and let it cool. The falling
temperature lowers the nucleation barriers of both the quasicrystalline
and crystalline phases. But because the liquid and quasicrystal phases have similar order--and hence a smaller
γ--the quasicrystalline phase has the lower
barrier and will solidify first. Eventually, the temperature drops to
the point that the second barrier is low enough for the metastable
phase to hop over and form the crystal.
Ken Kelton of
Electrostatic
levitation
Undercooling liquid metals is difficult. Even if a sample is
free from impurities, any bump or crevice on the walls of the vessel that
contains it can nucleate the solid phase at the equilibrium melting
temperature.
Turnbull and Cech
addressed the container problem by melting samples on flakes of amorphous
silica. They assumed that the amorphous substrate would be a poor nucleator of crystalline structure. But ideally, one
dispenses with a container. Thanks to surface tension, a drop of molten metal
holds itself together. So, to achieve the containerless
ideal, one levitates the drop and, for tracking structural changes, keeps it
motionless in a beam of x rays or neutrons.
|
Schematic diagram of
the electrostatic levitation chamber installed in an x-ray beamline. (Courtesy of Ken Kelton.) |
Several levitation methods exist. Kelton opted for electrostatic levitation and, for help,
turned to Jan Rogers of NASA's Marshall Space Flight Center in
Before electrostatic levitation can begin,
the initially solid drop is charged by induction. Electrodes above and below
the drop create the levitation field, which, being electrostatic, lacks minima.
Keeping the drop in place, therefore, is like balancing an upended broom: It
requires an active feedback system. The
A laser melts the drop, which cools radiatively. The drop's thermal radiation spectrum provides
the temperature diagnostic.
Recalescence
Kelton's original plan was to study
titanium-zirconium-nickel. The alloy forms metastable
icosahedral quasicrystals,
but Kelton was focusing instead on the alloy's stable
crystalline phase, a complex polytetrahedral
arrangement called C14 Laves.
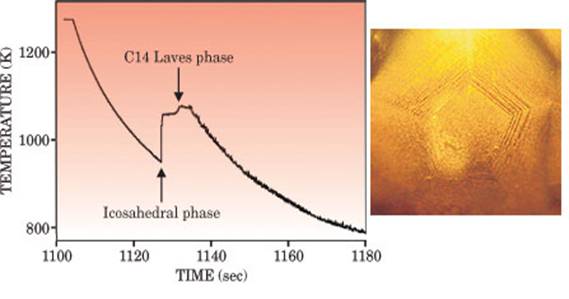
|
As a molten drop cools, its
temperature rises sharply at two specific phase transitions (left). First,
when the liquid forms the metastable icosahedral phase, which is quasicrystalline,
and later when it forms the C14 Laves phase, which is crystalline. An optical
mircograph (right) of the 2-mm-sized drop in its metastable state reveals pentagonal ridges. (Courtesy of
Ken Kelton.) |
In preliminary levitation experiments at
Kelton suspected that the first recalescence
signaled the formation of the alloy's metastable icosahedral phase, followed five seconds later by the
formation of the C14 Laves phase. Viewing the metastable
phase through an optical microscope confirmed its fivefold symmetry (figure 2). Here, Kelton realized,
was a likely material for testing Frank's hypothesis.
Confirming Frank's hypothesis involves not
only undercooling the right material, but also
measuring its atomic structure. And that involved a trip to the Advanced Photon
Source at Argonne National Laboratory in
A so-called third-generation synchrotron
source, APS produces x rays of high brightness and high energy. Both qualities
were invaluable for Kelton's experiment: The
brightness made it possible to collect data with high signal-to-noise on the
few-second timescale of the solidification, while the energies (125 keV, λ = 0.99 Å) made it possible to do a transmission
experiment rather than a more difficult reflection experiment.
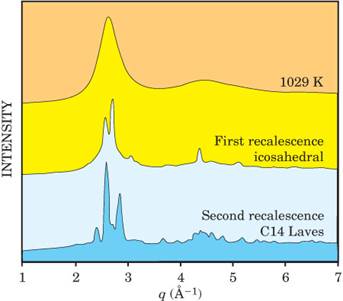
figure
3 shows three representative diffraction patterns taken at different stages
after the laser had melted the drop. The peaks appeared in the right places for
both the solid icosahedral and C14 Laves phases.
Frank was vindicated.
Nucleation
theory
|
X-ray diffraction patterns capture the structural changes
as the molten alloy (top) cools to solidify first into the icosahedral phase (middle) and then to the C14 Laves
phase (bottom). The peaks occur at the predicted locations and are plotted as
a function of the momentum transfer q
= 4π sinθ/λ, where θ is
the scattering angle and λ is the x-ray wavelength. (Adapted from ref. 2.) |
Figure 3 captures
snapshots of the two solid phases, but Kelton and his
colleagues could also obtain diffraction patterns at various points along the
cooling curve. That's especially interesting for comparing experiment with
theories of how crystals form.
Diffraction patterns depend on
experimental setup. To compare experiment with theory, one calculates structure
factors S(q), where q is the
momentum transfer. Constructing S(q)
from data involves modeling various aspects of the experiment, such as the
transmission of the levitation chamber's beryllium windows. Constructing S(q) from theory involves choosing an interatomic potential then doing either a large-scale
computer simulation or an approximate theoretical analysis.
In the early 1980s, before the discovery
of quasicrystals, Frank's ideas about local icosahedral ordering were applied to the formation of
metallic glasses.
The existence of icosahedral
order in the solidifying liquid has implications for classical nucleation
theory. In that picture, nucleation starts, or fails to start, in small
volumes. When the volume occupied by the nucleating phase exceeds the so-called
critical volume, fluctuations favor the formation of the new phase.
From his data, Kelton
derived both the size of the icosahedral clusters in
the liquid and the critical volume. Both turned out to be a few nanometers
across. The similarity of the two scales suggests that a liquid metal isn't a
structural blank slate. Structural correlations in the liquid could affect
crystallization.
The small scale of the critical volume
reveals a limitation of classical theory. When the crystallizing action takes
place on the scale of a few tens of atoms, it's unlikely that a clear-cut,
classical interface is appropriate. The challenge is to make nucleation theory
more atomistic.
Other
levitations, other systems
That a single system,
Ti-Zr-Ni, was observed to form a quasicrystalline
phase and then a crystalline phase was the key to proving Frank's hypothesis. But the 50-year-old theory had received impressive
support from similar work done by other groups.
The first to study the structure of
levitated drops were Dirk Holland-Moritz of the German Aerospace Research
Establishment (DLR) in
Ten years ago, the DLR team showed that
systems that have a high degree of icosahedral order
in the solid phase can be undercooled further than
systems that lack or have less icosahedral order.5
And last year, the DLR team and their
collaborators from two French institutions--Paris-Sud
University and the Center for Nuclear Studies in Grenoble--demonstrated
for four elemental metals and three alloys that the further a liquid undercools, the greater its icosahedral
order.6
Charles Day
References
1. D. Turnbull, R. E. Cech, J. Appl. Phys. 21,
804 (1950).
2. F. C. Frank, Proc.
R. Soc. London, Ser. A 215, 43 (1952).
3. K. F. Kelton et al., Phys. Rev. Lett. 90, 195504 (2003).
4. S. Sachdev, D. R. Nelson, Phys. Rev. Lett. 53, 1947 (1984) .
5. D. Holland-Moritz, D. M. Herlach, K. Urban, Phys. Rev. Lett. 71, 1196 (1993) .
6. T. Schenk et al., Phys. Rev. Lett. 89, 075507 (2002) .